UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| |
||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01 | Regulation FD Disclosure. |
On September 13, 2022, Better Therapeutics, Inc. (the “Company”) presented a corporate presentation at the H.C. Wainwright 24th Annual Global Investment Conference. A copy of the corporate presentation is filed as Exhibit 99.1 to this Current Report on Form 8-K. The corporate presentation will also be available on the Company’s website in the Investors section at https://investors.bettertx.com/news-events/events-presentations.
The information contained in Item 7.01 in this Current Report on Form 8-K and Exhibit 99.1 attached hereto is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit Number |
Description | |
| 99.1 | Corporate Presentation of Better Therapeutics, Inc., dated September 13, 2022. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Better Therapeutics, Inc. | ||||||
| Dated: September 13, 2022 | By: | /s/ Mark Heinen | ||||
| Name: | Mark Heinen | |||||
| Title: | Interim Chief Financial Officer | |||||

Exhibit 99.1 Pioneering Prescription Digital Therapeutics for Cardiometabolic Diseases SEPTEMBER 2022

Disclaimer This presentation (“Presentation”) is for informational purposes only. The information contained herein does not purport to be all-inclusive and neither Better Therapeutics, Inc. (“BetterTX” or the Company“) nor any of its respective affiliates nor any of its or their control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation. You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any decision. The reader shall not rely upon any statement, representation or warranty made by any other person, firm or corporation in making its investment or decision to invest in the Company. Neither the Company nor any of its respective affiliates nor any of its or their control persons, officers, directors, employees or representatives, shall be liable to the reader for any information set forth herein or any action taken or not taken by any reader, including any investment in shares of the Company. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and the Company’s own internal estimates and research. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while the Company believes its internal research is reliable, such research has not been verified by any independent source. This meeting and any information communicated at this meeting are strictly confidential and should not be discussed outside your organization. Forward-Looking Statements. Certain statements in this Presentation may be considered forward-looking statements, within the meaning of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements are typically identified by words such as “plan,” “believe,” “expect,” “anticipate,” “intend,” “outlook,” “estimate,” “forecast,” “project,” “continue,” “could,” “may,” “might,” “possible,” “potential,” “predict,” “should,” “would” and other similar words and expressions, but the absence of these words does not mean that a statement is not forward-looking. The forward-looking statements in this Presentation include, but are not limited to, statements regarding the delivery of cognitive behavioral therapy and/or prescription digital therapeutics by the Company to address the root causes of type 2 diabetes and other cardio metabolic diseases, development of a proprietary platform and software-based solutions for treatment of type 2 diabetes, heart disease and other conditions, achievement of changes in neural pathways of the brain and lasting changes in behavior through cognitive behavioral therapy delivered by the Company’s PDT, the capability of the Company to address the underlying causes of certain diseases and its related potential to improve patient health while lowering healthcare costs, the results of the trial of BT-001 in patients with type 2 diabetes, the Company's plans regarding FDA submissions, plans and expectations regarding the commercialization of BT-001, if approved, expectations related to the potential benefits of BT-001 and CBT and their potential treatment applications, the Company's plans regarding the research and advancement of its product candidates for additional treatments, expectations related to the interest of healthcare providers and payers in PDTs, including BT-001, the future financial stability, strength, or success of the Company, and legislative developments affecting PDTs and the outcome of such developments, among others. These forward-looking statements are based on the current expectations of the management of the Company and are inherently subject to uncertainties and changes in circumstances and their potential effects and speak only as of the date of such statement. There can be no assurance that future developments will be those that have been anticipated. These forward-looking statements involve a number of risks, uncertainties or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements including: risks related to the Company's business, such as the willingness of the FDA to authorize PDTs, including BT-001, for commercial distribution and insurance companies to reimburse their use, market acceptance of PDTs, including BT-001, the risk that the results of previously conducted studies will not be repeated or observed in ongoing or future studies involving our product candidates and other risks and uncertainties included under the header “Risk Factors” in the Company’s quarterly report on Form-10-Q for the fiscal quarter ended June 30, 2022 filed with the Securities and Exchange Commission (“SEC”) on August 11, 2022, and those that are included in any of the Company’s subsequent filings with the SEC. 2

The Problem Better Therapeutics Approach How our Product Works Contents BT-001 Pivotal Trial Go-to-Market Plan Investment Summary 3

% % Tn 50 86 $ 4.1 of adults in the US of healthcare dollars are spent on US National Health 2 2 have a chronic disease chronic disease maintenance 1 Expenditure in 2020 Currently available drugs treat symptoms but do not impact the root cause of disease. Most patients get worse over time despite being on multiple prescription drugs Sources: 1. Centers for Medicare and Medicaid Services; 2. Holman HR. The Relation of the Chronic Disease Epidemic to the Health Care Crisis. ACR Open Rheumatol. 2020 4 Mar;2(3):167-173. doi: 10.1002/acr2.11114. Epub 2020 Feb 19. PMID: 32073759; PMCID: PMC7077778.

We are spending more and more money to get worse and worse outcomes HYPERTENSION DIABETES US Healthcare Expenditures ($ in trillions) Americans Diagnosed with Diabetes (millions) US Healthcare Expenditures ($ in trillions) Americans Diagnosed with Hypertension (millions) $4 $4 110 30 $3 82.5 55 $2 22.5 $3 27.5 $1 0 1965 1975 1985 1995 2005 2015 $2 15 OBESITY US Healthcare Expenditures ($ in trillions) Americans Diagnosed with Obesity (millions) 100 $4 75 $3 $1 7.5 50 $2 25 $1 0 0 1965 1975 1985 1995 2005 2015 1965 1975 1985 1995 2005 2015 5
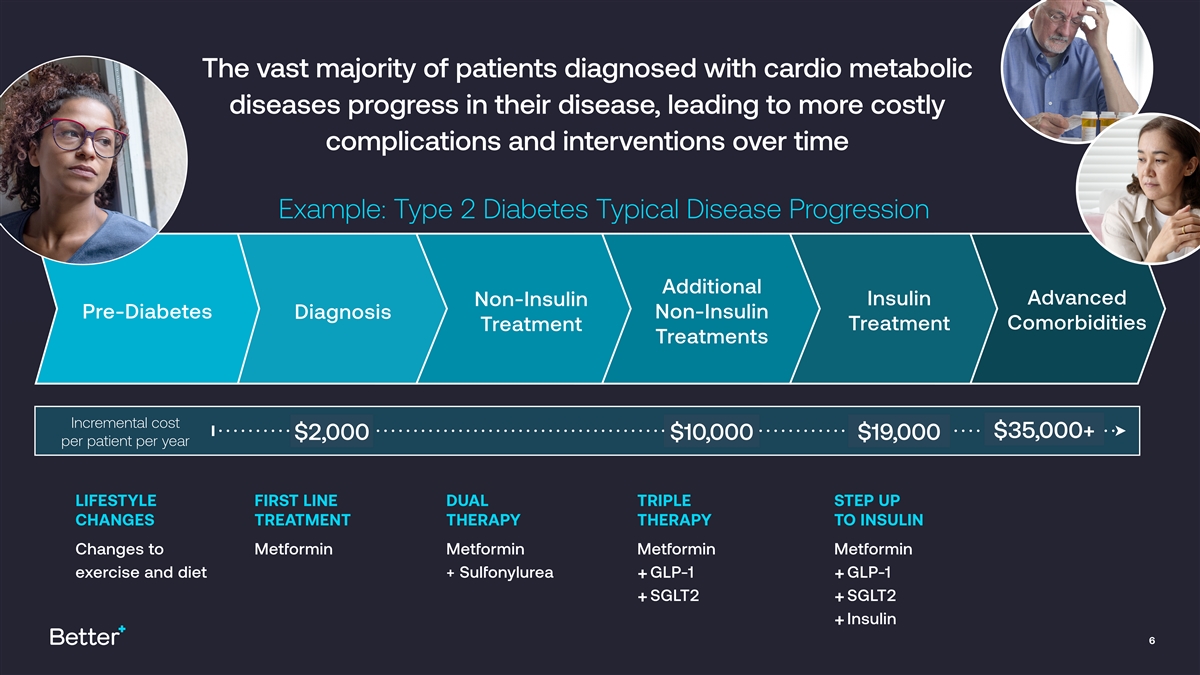
The vast majority of patients diagnosed with cardio metabolic diseases progress in their disease, leading to more costly complications and interventions over time 1 Example: Type 2 Diabetes Typical Disease Progression Additional Advanced Insulin Non-Insulin Pre-Diabetes Non-Insulin Diagnosis Comorbidities Treatment Treatment Treatments Incremental cost $35,000+ $2,000 $10,000 $19,000 per patient per year LIFESTYLE FIRST LINE DUAL TRIPLE STEP UP CHANGES TREATMENT THERAPY THERAPY TO INSULIN Changes to Metformin Metformin Metformin Metformin exercise and diet + Sulfonylurea GLP-1 GLP-1 + + SGLT2 SGLT2 + + Insulin + 6

The Problem Better Therapeutics Approach How our Product Works Contents BT-001 Pivotal Trial Go-to-Market Plan Investment Summary 7

Many of the most common (and costly) chronic diseases share lifestyle behaviors as a common root cause. Better Therapeutics combines medical, behavioral and digital/data science to create software based therapeutics that are designed to improve human health by targeting behavior change. 8

CURRENT CLINICAL GUIDELINES highlight behavior change as the foundation of treatment, but physicians have no options to prescribe it Emphasize importance of behavior change Call for digital solutions to facilitate behavior change Encourage reimbursement for solutions for behavior change No digital solutions available to prescribe behavioral therapy to treat root causes of diabetes and other cardiometabolic conditions 9

Next Generation Therapeutics: The Better Therapeutics Approach Developing Digital Therapeutics that are authorized by the FDA for a specific indication and have labeled claims. If authorized or cleared, patients obtain access through a physician prescription that is reimbursed via health insurance Initially focused on cardiometabolic diseases, which rank among the most common and costly chronic conditions that share lifestyle behaviors as a common root cause Advancing principles of Cognitive Behavioral Therapy (CBT), a well proven, validated approach to improve behavior by developing a novel CBT protocol and making it digitally available to improve access and scalability Rigorous product development incorporating patient & provider feedback into thoughtfully designed randomized controlled studies, backed up by Real World Evidence studies to support payer negotiations 10 CONFIDENTIAL

Opportunity to address healthcare inequities and access Digital therapeutics can reach patients where they are and connect them to the best care despite the many barriers patients experience Real-time insights into use and efficacy enables continuous improvement promising the potential for increasingly better efficacy without increased risk Using Software: Unique Benefits of Digital Data generated offers greater insights enabling better care, novel pricing models and continuous product improvement Therapeutics Development requires substantially less time and investment, enabling faster and more cost-efficient expansion into other potential indications or therapeutic areas 11

We are advancing a pipeline of PDT products using nCBT to treat multiple cardiometabolic diseases Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Q1 2023 Q2 2023 Q3 2023 Q4 2023 Pivotal (BT-001) De Novo Submission & FDA Review Type 2 Diabetes Pilot Hypertension Pilot Hyperlipidemia Discovery - LiVita Study Fatty Liver Disease (NASH/NAFLD) Pivotal BT-00X Additional Scientific Areas of Interest Increasingly, it is appreciated that there are shared pathways of pathophysiology, such as inflammation and immune activation that underlie the development of cardiometabolic conditions as well as conditions in other disease classes, such as Alzheimer’s disease, multiple sclerosis and certain cancers. 12

The Problem Better Therapeutics Approach How our Product Works Contents BT-001 Pivotal Trial Go-to-Market Plan Investment Summary 13

Cognitive Behavioral Therapy Developed in the 1960s, it is considered the gold standard for evidence-based therapy to help patients make lasting behavior changes by changing neural pathways in the brain 14

Traditional Cognitive Behavioral Therapy (CBT) is effective at addressing the behavioral root causes of cardiometabolic diseases but has limitations Not Standardized Not Scalable Treatment plans to treat cardiometabolic Patients must commit to 8 - 20 CBT diseases with CBT are selected by individual sessions with their healthcare 1 health professionals who have different levels professional. of success with their patients. Not Accessible Not Affordable Time and geographic constraints make in- Psychotherapists charge upwards of person sessions with a therapist $100/hr and not all patients have 2 impossible for many patients insurance that covers treatment. Sources: 1. Turner, J. The use of cognitive behavioral therapy in diabetes care: A review and case study. Journal of Diabetes Nursing 14, 3 (2010); Mayo Clinic Cognitive 15 Behavioral Therapy primer 2. Anxiety and Depression Association of America

We created nutritional CBT to treat the root causes of cardiometabolic diseases and designed it to be delivered digitally to make it accessible, affordable and scalable Works within the Unifies behavioral Goes far beyond the existing framework of therapy, lifestyle typical “cognitive standard medical care medicine, and AI into a distortions” in traditional and medication use. single therapeutic CBT to address eating experience and lifestyle behaviors 16

We deliver nutritional CBT using a mobile app prescribed by a physician Nutritional CBT is delivered via weekly therapy lessons, skill-building modules, and goal-setting. A treatment algorithm tailors treatment to each individual patient. Feedback is provided using a treatment score, rewards, and progress reports - to connect changes in behavior to improvements in blood sugar and other biometrics 17

COGNITIVE BEHAVIORAL THERAPY Changes thoughts and beliefs so that difficult behavior changes are possible. Changes neural pathways in the brain and builds the acceptance and resilience needed to handle challenging obstacles and emotions. 18

LIFESTYLE MEDICINE CBT creates the desire to change. Lifestyle medicine provides the “how.” Treatment Plan Guides changes in dietary behavior and physical activity, while improving medication adherence and self-monitoring. 19

ARTIFICIAL INTELLIGENCE We use AI to reveal the right treatment pace, intensity and support needed to maximize efficacy for each individual. Personalization A treatment algorithm dynamically adjusts goals to maximize treatment response, and provides a feedback loop that is designed to sustain engagement. 20

The data we collect helps patients get the best possible treatment experience and outcomes • Visualizations of treatment progress • Monitoring of engagement and biometrics • Alerts when patients disengage from treatment to enable early intervention • Algorithms predict patient success from engagement patterns 21

The Problem Better Therapeutics Approach How our Product Works Contents BT-001 Pivotal Trial Go-to-Market Plan Investment Summary 22

First in class, randomized control pivotal trial in Type 2 Diabetes BT-001 + Standard of Care Primary Endpoint Secondary Endpoint (Day 90) (Day 180) N = 325 Patients with Uncontrolled Type 2 Diabetes N = 668 Standard of Care Control Group N = 343 Mean change in Patients had longstanding type 2 Mean change in A1c reduction diabetes, were on multiple diabetes A1c reduction between BT-001 & medications, and had multiple between BT-001 Control Group comorbidities & Control Group Multiple additional exploratory endpoints 23

Nationally representative, diverse patient population Investigators mirror real-world prescribers Robust study design employed to minimize bias and set BT-001 Pivotal high comparison bar: Trial designed primarily for Control arm is Standard of Care (i.e. gold standard care), • not just treatment as usual FDA de novo Medication use and adjustment by investigators was not authorization • limited; only prandial insulin was excluded Patients were not mandated nor incentivized to use BT-001; • instead were free to self-select dose 24

BT-001 demonstrated clinically meaningful and sustained reduction in A1c over 180 days Both primary (A1c between group delta -0.4%, p<0.0001) and • secondary endpoint (A1c delta -0.3%, p=0.01) were met Significant improvements observed in BT-001 group despite use of • Pivotal Trial Results fewer diabetes medications Higher dose subgroups showed substantially greater A1c • improvements compared to control group Statistically significant Half of patients in BT-001 arm achieved absolute mean A1c reduction • & clinically meaningful of 1.3% (SD 0.8%) in this subgroup results in diverse Strong safety data, with significantly fewer Adverse Events in BT patient population arm (p<0.001) with advanced T2D BT-001 use associated with multiple additional cardiometabolic benefits and lower medication and healthcare utilization over time Patient engagement and persistence exceeded benchmarks for consumer health & wellness apps 25
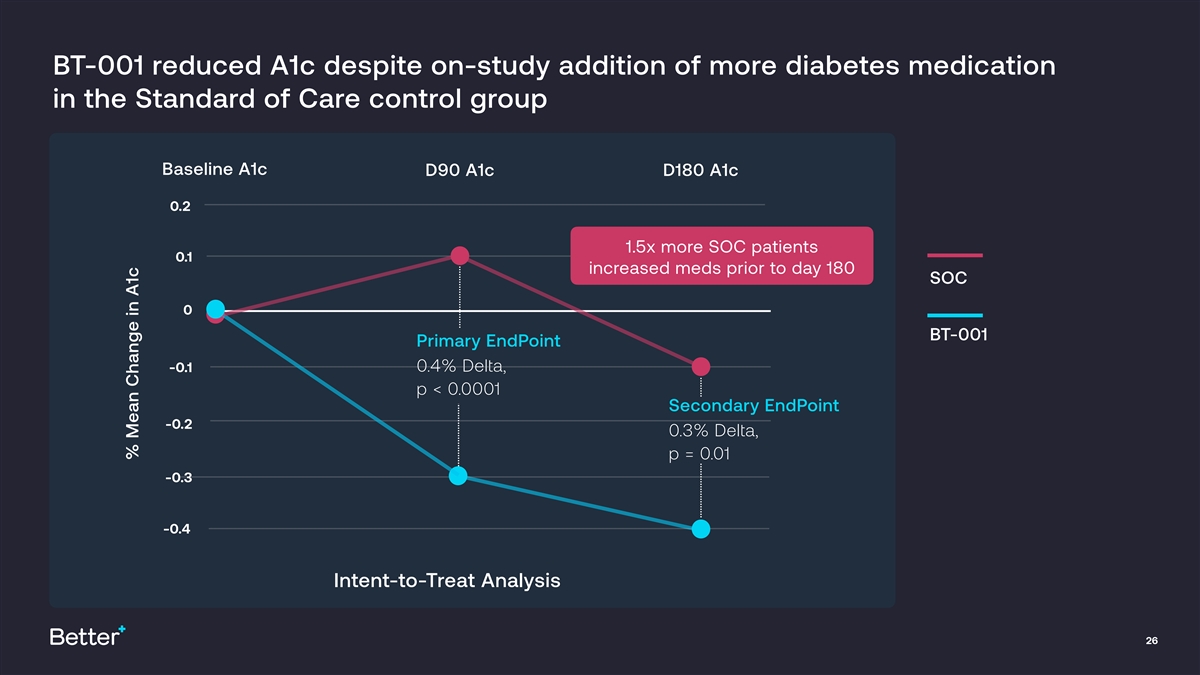
BT-001 reduced A1c despite on-study addition of more diabetes medication in the Standard of Care control group Baseline A1c D90 A1c D180 A1c 0.2 1.5x more SOC patients 0.1 increased meds prior to day 180 SOC 0 BT-001 Primary EndPoint 0.4% Delta, -0.1 p < 0.0001 Secondary EndPoint -0.2 0.3% Delta, p = 0.01 -0.3 -0.4 Intent-to-Treat Analysis 26 % Mean Change in A1c

Trending average change in fasting blood glucose in BT-001 group shows gradual and steady improvements, with no clear peak 27

Trends in fasting blood glucose in different therapies 0 -4 -8 -12 -16 -20 Baseline W4 W8 W12 W16 W20 W24 BT-001 Ferrannini E et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217-2224. Sitigliptin (GLP1) Goldstein BJ, et al; for Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30(8):1979–1987. Dapagliflozin (SGLT2) Note: These results are from different studies with different trial designs and patient populations. No head-to-head studies between these candidates have been conducted. 28 Fasting Blood Glucose Reduction (mg/dL)

Patients who used BT-001 more had greater reduction in A1c Participants self-selected dose of nCBT. Higher dose of nCBT lessons completed associated with larger A1c improvements at 180 days Low Use of Moderate Use of High Use of Behavior Therapy Behavior Therapy Behavior Therapy < 10 lessons 10-20 lessons >20 lessons 0 -0.1 -0.1% -0.2 -0.3 -0.4 -0.4% -0.5 -0.6 -0.6% -0.7 29 % Mean Change in A1c

1.5x more BT-001 patients achieved meaningful A1c change Significant improvements observed in BT-001 Group despite use of fewer diabetes medications ITT Population at D180 Δ = 16.1% p = 0.0004 Achieve 1% or more % 0 A1c reduction 30 (vs 17%, p=0.001) 10 20 30 34.3% 40 Achieve blood sugar % 50 control target of A1c < 7% 30 50.4% (vs 20% SOC, p=0.009) BT-001 Standard of Care 30 30 % Patients with 0.4% or more decrease

BT-001 Meaningful Responders show range of large improvements at 180 days Meaningful Responders” defined as 0.4% or more A1c improvement MEAN -1.3% MEDIAN 12% -1.1% 10% 8% 6% 4% 2% 0% -0.4 -0.5 -0.6 -0.7 -0.8 -0.9 -1 -1.1 -1.2 -1.3 -1.4 -1.5 -1.6 -1.7 -1.8 -1.9 -2 -2.1 -2.2 -2.3 -2.4 -2.5 -2.6 -2.7 -2.8 -2.9 -3 -3.1 -3.2 -3.3 <-3.3 D180 A1c Change From Baseline (% HbA1c) 31 % of BT-001 patients

180 Day safety data reveals significantly fewer Adverse Events BT-001 patients had statistically significant fewer AEs and Serious AEs Standard of Care BT-001 (n=343) (n=325) Subjects Events Subjects Events Number of subjects who experienced: n (%) n n (%) n An Adverse Event (AE) 188 (54.8%) 324 135 (41.5%) 265 p < 0.001 A Serious Adverse Event 24 (7.0%) 26 9 (2.8%) 9 p = 0.01 An AE Possibly/Probably Related to Study 0 (0.0%) 0 3 (0.9%) 4 Intervention An AE that is Related to Medical Software 0 (0.0%) 0 0 (0.0%) 0 BT-001 patients avoided more Serious Adverse Events (SAEs) commonly found in type 2 diabetes 32

Safety profiles of top performing diabetes drugs differ from BT-001 Adverse Reaction (>/= 5%) GLP1 SGLT2 BT-001 Pivotal Nausea Yes No No Vomiting Yes No No Diarrhea Yes No No Abdominal pain Yes No No Constipation Yes No No Female genital mycotic infections No Yes No Urinary track infections No Yes No Devise related adverse events N/A N/A < 1% Note: These results are from different studies with different trial designs and patient populations. No head-to-head studies between these candidates have been conducted. 33

Exploratory Endpoints Statistically significant findings in: Data revealed statistically significant Systolic Blood Pressure • changes in multiple Weight Reduction • endpoints, Mood Scores • underscoring potential Quality of Life Scores (Physical Health-Related) • for broad-based Adverse Event and Serious Adverse Event Rates • benefits Data to be submitted for peer-review publications. 34

During 180 days of use, patient engagement and persistence exceeded benchmarks for consumer health & wellness apps* 5.9 81% 61 Average minutes / day NPS Score after Percentage of patients spent in app using the app at 180 days 180 days % % % % 36 38 36 35 Healthcare Medical Fitness Health (all) Insurance 35 *Apptentive | 2022 Mobile Customer Engagement Benchmark Report - % retention at 90 days. Retention was 94% in BT-001 group at 90 days

The evidence from our pivotal trial suggests that BT-001 has the potential to be… We envision As efficacious as the best available drugs • BT-001 to become Safe - with generally fewer AEs and more use • standard of care not reflecting any increased risk for most adult Impactful on several health outcome measures • patients with T2D Beneficial from a health economics perspective • Accessible broadly to anyone with a • smartphone 36
The Problem Better Therapeutics Approach How our Product Works Contents BT-001 Pivotal Trial Go-to-Market Plan Investment Summary 37

Hypertension (High blood pressure) 70 million Type 2 Diabetes people suffering (High blood sugar) $30 billion 35 million people suffering Rx drug spending Our Digital $52 billion Therapeutics platform Root Causes Rx drug spending targets some of the Poor diet • Sedentary lifestyle • most prevalent and Stress • Poor sleep • costly conditions in Hyperlipidemia Alcohol, Tobacco • (High cholesterol) healthcare NASH / NAFLD (Non-alcoholic fatty liver disease) 40 million people suffering 64 million people suffering $28 billion Rx drug spending $100 billion Direct Healthcare Costs 38

PATH TO REGULATORY APPROVAL We plan to focus on securing coverage from regionally dominant, early adopting commercial payers, IDNs/health systems LEADING INDICATORS OF ADOPTION: PAYERS HEALTH SYSTEMS • Population health focused • Centralized decision-making • History of adopting new • Accountable Care technologies Organization (ACO) affiliations 39 39

Payers highlighted the following as meaningful and compelling • Efficacy, particularly in responders and improvement from 90 days to 180 days • Study design including patient diversity, robust treatment background, patient self selection of dose Payer response to • Patient retention at 180 days; better than drug compliance pivotal data has been • Secondary endpoint results positive • Potential for cost offsets In research conducted with current or recent senior level Areas discussed that are going to be addressed with ongoing RWE studies, decision makers from national implementation and innovative contracting/pricing payers, PBMs, regional payers, and health systems/payers (n=6) • Durability of response over time after treatment (1 year) • Number of lessons needed for meaningful response • Prediction of patient response based on early engagement patterns 40

Payer Survey National and regional payers, as well as PBMs reacted positively to BT-001’s Target Product Profile Likelihood to Cover BT-001 (n=14) 71% responded “likely to cover” 1 2 3 4 14% 14% 43% 21% 7% 5 6 7 Source: Better Therapeutics Quantitative Market Research, Feb/Mar 2022. The 1 to 7 scale represents the likelihood of the 41 payer to reimburse BT-001 to at least some of their patients, where 1 was “not at all likely” and 7 was “extremely likely.”

Provider Survey Providers have expressed a willingness to prescribe BT-001 based on Target Product Profile Likelihood to Prescribe BT-001 (n=25) 88% rated “likely to prescribe” 1 2 3 4 4% 8% 12% 48% 28% 5 6 7 Source: Better Therapeutics Quantitative Market Research, Feb/Mar 2022. The 1 to 7 scale represents the likelihood of the 42 provider to prescribe BT-001 to at least some of their patients, where 1 was “not at all likely” and 7 was “extremely likely.”

The Problem Better Therapeutics Approach How our Product Works Contents BT-001 Pivotal Trial Go-to-Market Plan Investment Summary 43

De Novo Submission Q3 2022 Upcoming Milestones Initiate payer discussions Q3 2022 Top line data from LivVita Liver Study for Q4 2022 NAFLD and NASH Q4 2022/ Address financing needs Q1 2023 Pending FDA Commercial launch Authorization Pipeline Expansion / Next Pivotal Study 2023 44

LivVita Study Top-Line Data expected in Q4 2022 Measured at 90 Days Primary Endpoint Average change in liver fat after 90 days in participants with baseline proton density fat fraction (PDFF) ≥ 10%, as Single Arm Interventional measured by MRI-PDFF Cohort Study Patients with non-alcoholic steatohepatitis (NASH) and Secondary Endpoints Average change in liver fat from baseline to end of treatment in all non-alcoholic fatty liver participants and percent who achieve ≥ 30% reduction in PDFF disease (NAFLD). Exploratory Endpoints N = 22 Various clinical markers of liver and cardiometabolic health, along with quantitative feedback from participants to inform future clinical product development in these indications. 45

Investment in BTTX - Favorable Risk-Return Profile Potential to disrupt and create substantial value Targeting some of the largest indications in healthcare Addressing significant unmet medical needs and massive expense burdens Strong pivotal data - Impact on several health outcome measures Potential to not just impact symptoms but change/reverse the course of disease Good for patients, providers and payers Efficacy, safety & accessibility plus alignment with current treatment guidelines Beneficial from a health economics perspective Ability to expand pipeline faster & with significantly less investment than traditional pharma Potentially higher profitability business model than traditional pharma 46

Pioneering Prescription Digital Therapeutics for Cardiometabolic Diseases
