UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01 | Regulation FD Disclosure. |
On December 8, 2022, Better Therapeutics, Inc. (the “Company”) announced positive topline results from its LivVita liver study evaluating the feasibility of cognitive behavioral therapy to reduce liver fat and improve liver disease biomarkers as a potential treatment for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. A copy of the press release is being furnished as Exhibit 99.1 to this Current Report on Form 8-K.
On December 8, 2022, the Company issued a corporate presentation on the LivVita liver study results for its investor call. A copy of the corporate presentation is being furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information contained in Item 7.01 in this Current Report on Form 8-K and Exhibit 99.1 and Exhibit 99.2 attached hereto is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit Number |
Description | |
| 99.1 | Press Release of Better Therapeutics, Inc., dated December 8, 2022. | |
| 99.2 | Corporate Presentation of Better Therapeutics, Inc., dated December 8, 2022. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Better Therapeutics, Inc. | ||||||
| Dated: December 9, 2022 | By: | /s/ Mark Heinen | ||||
| Name: | Mark Heinen | |||||
| Title: | Interim Chief Financial Officer | |||||
Exhibit 99.1
Better Therapeutics Completes Exploratory Trial for Fatty Liver Disease and Announces Positive Topline Results
Study Conducted with Arizona Liver Health Met Primary Endpoint and Established Proof-of-Concept for Prescription Digital Therapy Platform to Improve Disease Biomarkers in NAFLD and NASH Patients
Company Intends to Apply for Breakthrough Device Designation with FDA
Company to Host Conference Call and Webcast Today at 8:30 a.m. ET
SAN FRANCISCO, December 8, 2022 – Better Therapeutics, Inc. (NASDAQ: BTTX), a prescription digital therapeutics (PDT) company developing cognitive behavioral therapy (CBT) to address the root causes of cardiometabolic diseases, today announced positive topline results of the first ever study evaluating the feasibility of using a prescription digital therapeutic to reduce liver fat and improve liver disease biomarkers in Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH).
The study included a cohort of 22 patients with NAFLD and NASH and used Magnetic Resonance Imaging - Proton Density Fat Fraction (MRI-PDFF) scans, a validated proxy for liver biopsies, to monitor changes in liver fat. Changes in a range of exploratory liver biomarkers were also assessed. Currently, there is no FDA approved treatment for these conditions, which affect approximately one in four Americans and cause approximately $100 billion in direct medical cost annually. And while some drug candidates are in various phases of research and development, they often present side effects that could limit their utility in patients, if approved.
“With approximately 90 million Americans affected by NAFLD, fatty liver disease is a public health crisis that has hit epidemic levels. Because there are currently no FDA approved therapies available to treat these patients, we need to evaluate every possible option to reduce the burden of this condition – and in this study we have found positive topline signals across multiple disease biomarkers for a potential new option in our fight against NAFLD and NASH,” said Mazen Noureddin, MD, Director of the Houston Liver Institute and who serves as the Senior Scientific Advisor for the LivVita Study. “Since the cause and progression of these conditions are linked to behavioral factors like diet and activity level, the utilization of a prescription digital therapy that targets these behaviors is compelling and appears to have the potential to make a real difference in this costly disease.”
The LivVita Study met its primary endpoint, showing a statistically significant positive signal with an average relative reduction in MRI-PDFF of 16% (p=0.01) in the intent-to-treat population (N=19). Additional highlights include:
| • | A statistically significant mean reduction in Alanine transaminase (ALT) of -17 IU/L (p=0.002) |
| • | A statistically significant mean change in FAST Score of 20% (p=0.01) |
| • | No severe adverse events or device related adverse events |
| • | High engagement and patient satisfaction with treatment, with Net Promoter Score of +75 and 94% of subjects still using the app after 90 days |
“The results seen in the LivVita Study give us confidence that there is merit in further development of treatments for NASH and NAFLD that leverage digital therapies and CBT techniques,” said Naim Alkhouri, MD Director of the Fatty Liver Program at Arizona Liver Health and Principal Investigator of the study. “We have long understood that making real changes to certain behaviors can result in slowed progression of this disease, but we have not had a reliable, scalable way to deliver the support people need to make them. If we can use smartphones as a delivery mechanism for meaningful therapy, it should certainly become a tool we lean on as we help patients live with these chronic conditions.”
The study relied on Better Therapeutics’ CBT platform, which has been developed with the intention of helping patients with cardiometabolic diseases – including NASH and NAFLD – access a tailored treatment that leverages CBT techniques to address underlying causes of these diseases and help them make sustainable behavioral changes. This platform has already shown progress elsewhere, leading to the submission of a de novo classification request for BT-001, Better Therapeutics’ prescription digital therapy for type 2 diabetes, currently under review by the FDA.
“In our initial work on BT-001, we have already seen indications that our novel form of CBT delivered through a prescription digital therapeutic may help with cardiometabolic conditions. The encouraging early results from our work with fatty liver disease continues to build that body of evidence,” said Mark Berman, MD CMO of Better Therapeutics. “As we move towards 2023 and the anticipated launch of our first digital therapeutic, pending FDA authorization, we are more confident than ever in the promise of this approach to treating these conditions.”
Better Therapeutics intends to publish these data in a peer-reviewed journal, apply for breakthrough device designation with the FDA, and potentially seek a partner to accelerate development of a NAFLD/NASH specific PDT.
Conference Call and Webcast
Better Therapeutics will host a conference call and webcast today, December 8, 2022, at 8:30 a.m. ET / 5:30 a.m. PT to review the topline results from the LivVita Study. To access the conference call, please register at: https://register.vevent.com/register/BI2e2fa3ed900e4fa49b51635011507657. Upon registering, each participant will be provided with call details and access codes. All participants are encouraged to join 10 minutes prior to the start time. The live webcast may be accessed by visiting the event link at: https://edge.media-server.com/mmc/p/xpog3h3b. A replay of the webcast may be accessed from the Presentations & Events page in the Investors section of the Better Therapeutics corporate website at: investors.bettertx.com.
About Better Therapeutics
Better Therapeutics is a prescription digital therapeutics (PDT) company developing a novel form of cognitive behavioral therapy (CBT) to address the root causes of cardiometabolic diseases. The company has developed a proprietary platform for the development of FDA-regulated, software-based solutions for type 2 diabetes, heart disease and other conditions. The CBT delivered by Better Therapeutics’ PDT is designed to enable changes in neural pathways of the brain so lasting changes in behavior become possible. Addressing the underlying causes of these diseases has the potential to dramatically improve patient health while lowering healthcare costs. Better Therapeutics’ clinically validated mobile applications, if authorized for marketing, are intended to be prescribed by physicians and reimbursed like traditional medicines.
For more information visit: bettertx.com
Forward-Looking Statements
Certain statements made in this press release are “forward-looking statements” within the meaning of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements are typically identified by words such as “plan,” “believe,” “expect,” “anticipate,” “intend,” “outlook,” “estimate,” “forecast,” “project,” “continue,” “could,” “may,” “might,” “possible,” “potential,” “predict,” “should,” “would” and other similar words and expressions, but the absence of these words does not mean that a statement is not forward-looking. The forward-looking statements in this press release include, but are not limited to, statements regarding the delivery of CBT and /or PDTs by Better Therapeutics to address the root causes of NAFLD, NASH, type 2 diabetes and other conditions, Better Therapeutics’ plans regarding FDA submissions, expectations related to the potential benefits of BT-001 and CBT and their potential treatment applications and the limitations of other drug candidates to address the treatment of NAFLD and NASH, Better Therapeutics’ plans regarding the research and advancement of its PDTs for NAFLD, NASH and additional treatments, plans and expectations regarding the commercialization of BT-001, if approved, expectations related to the interest of healthcare providers and payers in PDTs and legislative developments affecting PDTs and the outcome of such developments, among others. These forward-looking statements are based on the current expectations of the management of Better Therapeutics and are inherently subject to uncertainties and changes in circumstances and their potential effects and speak only as of the date of such statement. There can be no assurance that future developments will be those that have been anticipated. These forward-looking statements involve a number of risks, uncertainties or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements including: risks related to Better Therapeutics’ business, such as the willingness of the FDA to authorize PDTs for commercial distribution and insurance companies to reimburse their use, market acceptance of PDTs, the risk that the results of previously conducted studies will not be repeated or observed in ongoing or future studies involving our product candidates and other risks and uncertainties included under the header “Risk Factors” in Better Therapeutics’ quarterly report on Form 10-Q for the quarter ended September 30, 2022 filed with the Securities and Exchange Commission (SEC) on November 14, 2022, and those that are included in any of Better Therapeutics’ subsequent filings with the SEC.
Exhibit 99.2

DECEMBER 8, 2022 LivVita Liver Health Feasibility Study Topline Data Better+ T HERA PEU TICS

Disclaimer This presentation (“Presentation”) is for informational purposes only. The information contained herein does not purport to be all-inclusive and neither Better Therapeutics, Inc. (“BetterTX” or the “Company”) nor any of its respective affiliates nor any of its or their control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation. You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any decision. The reader shall not rely upon any statement, representation or warranty made by any other person, firm or corporation in making its investment or decision to invest in the Company. Neither the Company nor any of its respective affiliates nor any of its or their control persons, officers, directors, employees or representatives, shall be liable to the reader for any information set forth herein or any action taken or not taken by any reader, including any investment in shares of the Company. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and the Company’s own internal estimates and research. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while the Company believes its internal research is reliable, such research has not been verified by any independent source. This meeting and any information communicated at this meeting are strictly confidential and should not be discussed outside your organization. Forward-Looking Statements. Certain statements in this Presentation may be considered forward-looking statements, within the meaning of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements are typically identified by words such as “plan,” “believe,” “expect,” “anticipate,” “intend,” “outlook,” “estimate,” “forecast,” “project,” “continue,” “could,” “may,” “might,” “possible,” “potential,” “predict,” “should,” “would” and other similar words and expressions, but the absence of these words does not mean that a statement is not forward-looking. The forward-looking statements in this Presentation include, but are not limited to, statements regarding the delivery of cognitive behavioral therapy and/or prescription digital therapeutics by the Company to address the root causes of NAFLD/NASH and other cardio metabolic diseases, development of a proprietary platform and software-based solutions for treatment of NAFLD/NASH and other conditions, achievement of changes in neural pathways of the brain and lasting changes in behavior through cognitive behavioral therapy delivered by the Company’s PDT, the capability of the Company to address the underlying causes of certain diseases and its related potential to improve patient health while lowering healthcare costs, the results of study of the Company’s PDT in patients with NAFLD and NASH, the Company’s plans regarding FDA submissions, partnership opportunities and publications, expectations related to the potential benefits of PDTs and CBT and their potential treatment applications and the limitations of other drug candidates to address the treatment of NAFLD and NASH, the Company’s plans regarding the research and advancement of its product candidates for additional treatments, expectations related to the interest of healthcare providers and payers in PDTs, the future financial stability, strength, or success of the Company, and legislative developments affecting PDTs and the outcome of such developments, among others. These forward-looking statements are based on the current expectations of the management of the Company and are inherently subject to uncertainties and changes in circumstances and their potential effects and speak only as of the date of such statement. There can be no assurance that future developments will be those that have been anticipated. These forward-looking statements involve a number of risks, uncertainties or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements including: risks related to the Company’s business, such as the willingness of the FDA to authorize PDTs for commercial distribution and insurance companies to reimburse their use, market acceptance of PDTs, the risk that the results of previously conducted studies will not be repeated or observed in ongoing or future studies involving our product candidates and other risks and uncertainties included under the header “Risk Factors” in the Company’s quarterly report on Form-10-Q for the fiscal quarter ended September 30, 2022 filed with the Securities and Exchange Commission (“SEC”) on November 14, 2022, and those that are included in any of the Company’s subsequent filings with the SEC. Better· 2

Better therapeutics Pioneering Prescription digital therapeutics for cardiometabolic diseases

Contents better NAFLD/NASH Overview LivVita Topline Results conclusions Next Steps

Non Alcoholic fatty liver Disease (NAFLD) includes a spectrum of conditions rooted in unhealthy lifestyle behaviors Fatty liver/ NASH+ Hepatocellular Cirrhosis NAFL Fibrosis Carcinoma Obesity Type 2 Diabetes Dyslipidemia Other factors Fat Fat plus Scar tissue Cancer Accumulation Inflammation, Replaces liver Only Scarring Cells Image source https:// pharmaintelligence.informa.com/resources/product-content/naas-flying-flying-the-plane-building-it better 5

NAFLD is at epidemic levels in the United states and is leading cause of liver transplantation1 30%of adults in US have NAFLD 5% of adult in us have NASH Better 6

NAFLD and NASH prevalence projected to increase significantly2 with a growing proportion of NAFLD patient progressing to NASh Cases in millions 110 82.5 55 27.5 0 2015 2030 NAFLD 2015 2030 NASH Better 7

At present, despite billions invested, there are no FDA approved pharmacological agents for the treatment of NAFLD or NASH And leading candidates have frequent side effects Diarrhea Nausea Vomitting Itching Pemvidutide3 GLP1jglucagon dual receptor agonist 22% 52% 9% Phase1b Efruxifermin4 Fibroblast growth factor 21 (FGF21) analog 33% 33% Phase 2b Semaglutide5 GLP1 analog 28% 37% 22% Phase 2b Resmetiroms THR {3-selective agonist 31% 18% Phase 2b Obeticolic acid? 51% FXR-agonist 7% 13% 7% (28% characterized as Phase 3 intense or widespread) Better· 8

We believe that addressing the unmet needs in NAFLD/NASH requ1res non-pharmacological therapies Qualities of an ideal new therapy in NAFLD Addresses full breadth of modifiable behavioral root causes Safe and well-tolerated Readily accessible, convenient and desired by patients Improves liver fat, glucose & lipid metabolism and inflammation Reduce cardiovascular risk associated with common comorbidities. Sustained effect Reduces Burden on providers

Contents NAFLD/NASH Overview LivVita Study Desingn Livvita Topline Results Conclusions Next Steps better 10

LivVita Study was designed to assess potential for both efficacy and safety in NAFLD and/or NASH Partnered with leading specialty liver health clinic—Arizona Liver Heath—to design study and recruit diverse set of participants in 2 sites BT’s cardiometabolic Cognitive Behavioral Therapy (CBT) research platform was evaluated over a 3-month treatment period Basic liver health education provided in 1 phone session prior to treatment MRI-PDFF and robust set of biomarkers evaluated, to potentially enable planning of pivotal study App-engagement and qualitative data collected, to inform customizations needed for liver-specific product
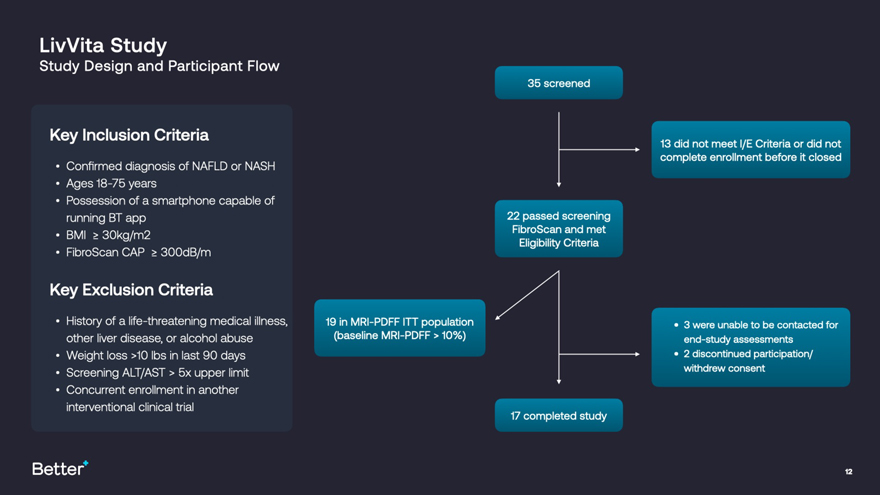
LivVita Study Study Design and Participant Flow 35 screened Kev Inclusion Criteria 7i djd meet Criteria Q|. did nQt complete enrollment before it closed Confirmed diagnosis of NAFLD or NASH Ages 18-75 years Possession of a smartphone capable of running BT app 22 passed screening BMI > 30kg/m2 Eligibility Criteria FibroScan CAP > 300dB/m Key Exclusion Criteria / History of a life-threatening medical illness, 19 in MRI-PDFFITT population .3 were unable to be contacted for other liver disease, or alcohol abuse (baseline MRI-PDFF > 10%) end-study assessments Weight loss >10 lbs in last 90 days 2 discontinued participation/ Screening ALT/AST > 5x upper limit withdrew consent Concurrent enrollment in another interventional clinical trial 17 completed study Better

LivVita Study Topline Key Endpoints Magnetic Resonance Imaging-Proton Density Fat Fraction (MRI-PDFF): A specialized type of MRI that provides the most validated and reproducible non-invasive quantitative measure of liver fat Alanine Transaminase (ALT): An enzyme produced by liver cells. ALT blood levels rise when the liver is damaged or injured FAST Score: A composite score the uses FibroScan’s measures of liver stiffness and fat combined with the Aspartate Transaminase (AST) blood test to predict degree of NASH risk FibroScan CAP Score: A quantitative measure of liver fat using a specialized type of ultrasound that provides point of care screening and assessment of liver fat and stiffness Net Promoter Score (NPS): A survey that measures user satisfaction and likelihood that the individual would recommend a product to a friend or family member. Scores range from -100 to +100 Better

NAFLD/NASH Overview LivVita Study Design Contents • LivVita Topline Results Conclusions Next Steps

Diverse set of patients recruited from 2 specialty liver clinics in Arizona Baseline characteristics represent commonly occurring cardiometabolic conditions in patients with NAFLD and NASH _ x > x Safety Population Parameter / Category (n=22) Age (mean) 48 yrs % Female 77% % Non-white 47% % Hispanic/Latino 41% Body Mass Index (mean) 38 kg/m2 Liver Disease Diagnosis at Baseline NASH 77% NAFLD 23% Baseline Liver Fat (mean MRI-PDFF %) 19% Number of Comorbidities (mean) 6 Type 2 Diabetes 46% Hypertension 59% Hyperlipidemia 41%

The primary endpoint, MRI-PDFF, showed positive signal with statistically significant reductions in liver fat (p = 0.01)

Percent Change in MRI-PDFF Intent-to-Treat Population “ â– -16% 18 Relative Reduction u. § 1 -11 -tl -12 -12 1R fl B a -16 _17 -is 0-26 L—so -64% Relative Reduction -70 ‘64 ALT results showed statistically significant reductions in marker of liver damage (p = 0.002)

FAST Score results showed statistically significant reductions in NASH Risk (p = 0.006)

FAST Score results showed statistically significant reductions in NASH Risk

CAP and Weight Change showed statistically significant reductions in markers of liver damage Change in CAP Score Change in Weight A = -19 dB/m (-6%) p= 0.021 A = -7 lbs (-3% TBW) p = 0.008

Trending average change in patient-reported weight showed gradual and steady improvements, with no clear peak

Dose response demonstrated positive relationship between CBT engagement and improvements in liver health biomarkers Low Use of Behavior Therapy High Use of Behavior Therapy < 13 Lessons completed >= 13 lessons completed

Safety data revealed no device related adverse events (AEs) were experienced BT’s CBT was generally well-tolerated with few overall AEs reported over 3-month period Safety Population (n=22) Number of subjects who Subjects Events .... . .. . . . /ozx Mild Moderate Severe experienced: n (%) n An Adverse Event (AE) 6(27%) 10 5 5 0 An AE Possibly/Probably Q Q Related to Study Intervention ° An AE that is Related to Medical Device ( o) Serious Adverse Event (SAE) 0 (0%) 0

During 90 days of use, patient engagement and satisfaction significantly exceeded benchmarks for consumer health & wellness apps8>9. Notable Quality of Life improvements also reported10 94% 2.2 +75 Percentage of subjects Average added Healthy IMPS Score after 90 days using the app in Week 12 Days/month reported Scored on a scale from -100 to +100 42% reported an improvement in 1 or more Quality of Life Category 38% 36% 35% +38 Medical Fitness Health Healthcare Industry Insurance Benchmark

Contents LivVita Topline Results • Conclusions Next Steps NAFLD/NASH Overview LivVita Study Design

LivVita Study demonstrated clear potential for response, with no safety related concerns observed, supporting our hypothesis and research platform Consistent improvements in MRI-PDFF and broad range of liver biomarkers found, with notable changes in ALT and FAST scores; improvements are consistent with our CBT’s broad mechanism of action Generally well-tolerated profile and excellent patient-satisfaction scores suggests great potential for patient-centric NAFLD/NASH treatment Potential exists to establish NAFLD/NASH PDT as part of standard of care treatment either alone or alongside future pharmacotherapy treatments _ Study also serves as proof-of-concept of BT’s indication discovery platform—which could afford for rapid ability to expand indication pipeline

Naim Alkhouri, MD Director of the Fatty Liver Program Arizona

Contents NAFLD/NASH Overview LivVita Study Design LivVita Topline Results Conclusions Next Steps

Next Steps • Submit manuscript for peer-reviewed publication _ Submit application to FDA for Breakthrough Device Designation and commence discussions on pivotal study design _ Consider partnership opportunities to accelerate development of BT’s NAFLD/NASH specific PDT

References Cotter TG, Rinella M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology. 2020 May;158(7):18511864. doi: 10.1053/j.gastro.2020.01.052. Epub 2020 Feb 13. PMID: 32061595. Estes, C., Razavi, H., Loomba, R., Younossi, Z., & Sanyal, A. J. (2018). Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology (Baltimore, Md.), 67(1), 123133. https://doi.org/10.1002/hep.29466 Altimmune Announces Significant Reductions in Liver Fat Content and Body Weight in 12Week Phase 1b Clinical Trial of Pemvidutide in Subjects with NAFLD—Altimmune. (September 14, 2022.). Retrieved November 29, 2022, from https://ir.altimmune.com/newsreleases/newsreleasedetails/altimmuneannounces significantreductionsliverfatcontentan d Akero Therapeutics. 2O22.“Phase 2b HARMONY Study Results,” September 12. https://ir.akerotx.com/staticfiles/b3cbfed13eee49cf8a10cc7500Q855d5 Newsome, P. N., Buchholtz, K., Cusi, K., Linder, M., Okanoue, T., Ratziu, V., Sanyal, A. J., Sejling, A.S., & Harrison, S. A. (2021). A PlaceboControlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. New England Journal of Medicine, 384(12), 11131124. https://doi.org/10.1056/NEJMoa2028395 Positive Topline Phase 3 MAESTRONAFLD1 Data Demonstrate Resmetirom was Safe, WellTolerated and Provided Statistically Significant Improvements in Key Measures of Liver and Cardiovascular Health | Madrigal Pharmaceuticals. (January 31, 2022.). Retrieved November 29, 2022, from https://ir.madrigalpharma.com/news releases/newsreleasedetails/positivetoplinephase3maestronafld1datademonstrate Younossi, Z. M., Ratziu, V., Loomba, R., Rinella, M., Anstee, Q. M., Goodman, Z., Bedossa, P., Geier, A., Beckebaum, S., Newsome, P. N., Sheridan, D., Sheikh, M. Y., Trotter, J., Knappie, W.» Lawitz, E., Abdelmalek, M. F., Kowdley, K. V., MontanoLoza, A. J., Boursier, J.,... Zuin, M. (2019). Obeticholic acid for the treatment of nonalcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebocontrolled phase 3 trial. The Lancet, 394(10215), 21842196. https://doi.org/10.1016/ S01406736(19)330417 2022 Mobile Customer Engagement Benchmark Report, (n.d.). Apptentive. Retrieved December 5, 2022, from https://www.apptentive.com/2022benchmarkreport/ What is a Good Net Promoter Score? (2022 NPS Benchmark). (2022, April 18). Retently. https://www.retently.com/blog/goodnetpromoterscore Healthy Days Core Module: HRQOL14 Measure | HRQOL | CDC. (2018, November 5). https://www.cdc.gov/hrgol/hrgol14_measure.htm
